Why only Lithium-Ion Batteries for Electric Vehicle Batteries
Electric car owners must inspect their vehicles many hours before going on another road trip. If you were an energy- and range-conscious driver, you would determine if your device still had enough power to last the distance. You’ll see from that perspective how crucial the battery is to your hassle-free, environmentally responsible driving. You would pick a car model that not only has the newest features and innovations but also has a battery that outperforms those from the top manufacturers.
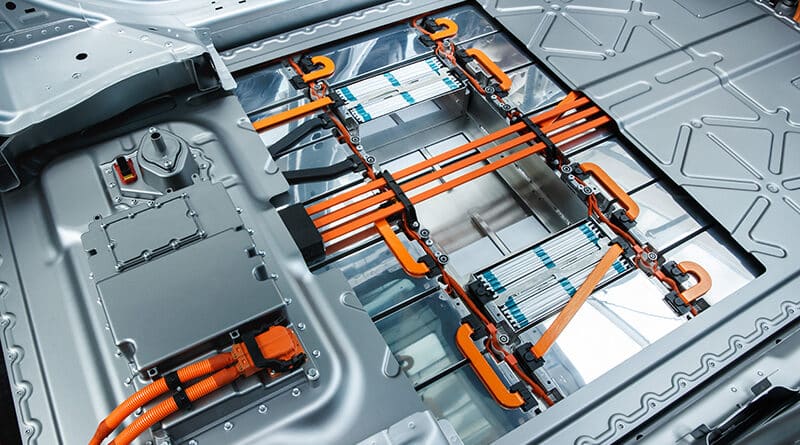
Most electric vehicles are powered by lithium-ion (li-on) batteries, the battery technology with the fastest growth and most significant promise. Lithium-ion batteries, an environmentally acceptable replacement for lead acid batteries, provide three times the energy density of a typical lead acid battery. The lighter and more durable lithium-ion battery can produce the same power with a higher energy density potential. Rechargeable lithium-ion batteries require little maintenance and are becoming more affordable.
Workings of lithium-ion batteries
Four primary components in a lithium-ion battery cell are constructed from different materials. Each component carries out its particular duties:
- Lithium metal oxide powder serves as the cathode, which transfers lithium ions to the anode during charging and receives them during discharging.
- Graphite powder is used to make the anode. It takes lithium ions from the cathode during charging and releases them during discharging.
- Between the cathode and the anode, the electrolyte transports lithium ions. Organic solvents and lithium salts are used to make this component.
- Separator: By keeping the cathode and the anode from touching, this part avoids short circuits. Lithium ions move via these pores at this point. Micro-porous membranes are used to create the separator.
The lithium ions migrate between the anode and the cathode to produce electricity flow for electronic devices. During the discharge cycle, the lithium in the anode is ionized and subsequently released into the electrolyte. Lithium ions enter the cathode’s holes after passing through the separator. At the same time, the anode releases electrons, creating the electric current that flows to an external electric circuit. The lithium ions shift from the cathode to the anode during the charge cycle, a chemical reaction that can reverse. The lithium-ion battery is recharged in this manner.
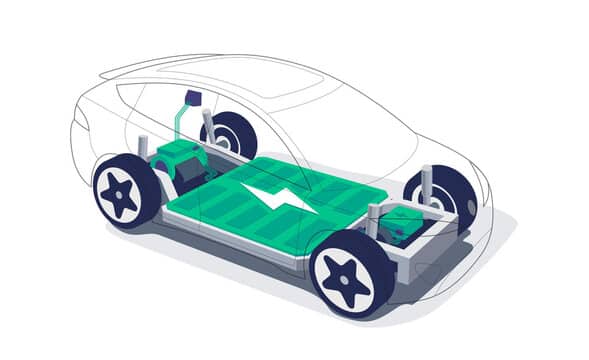
How electric vehicles started using lithium-ion batteries
The US always creates alternate fuels and renewable energy sources to power its automobiles. The main focus is on electricity, which is now produced by solar and wind power. Engineers are also considering using hydrogen and natural gas in power grids to meet the electricity demand from electric vehicles. Why did people use electricity instead of gasoline and other oil-based fuels? There are three primary causes.
- Reduce emissions of greenhouse gases.
- Reduce reliance on imported oil exports.
- Keep and add additional nearby jobs (reviving its automobile sector with an emphasis on electric vehicles and battery manufacturing).
All of them are related to electric vehicles, which the state and federal governments actively support through various financial incentives for car customers and requirements for automakers. The viability of electric vehicles will depend on batteries, which remain a crucial weakness. Engineers and scientists working in electric mobility are getting closer to creating an electric car battery that is safer, more potent, and uses less energy.
The majority of electric vehicles, as previously indicated, employ lithium-ion batteries. Lithium-ion batteries are predicted to overtake other types of batteries as consumers switch from internal combustion engines to electric motors in more significant numbers. These batteries are used in plug-in hybrid and fully electric automobiles. According to the Deutsche Bank in 2009, lithium-ion batteries will rule the market by 2017 despite lead acid and nickel metal batteries still holding a substantial market share.
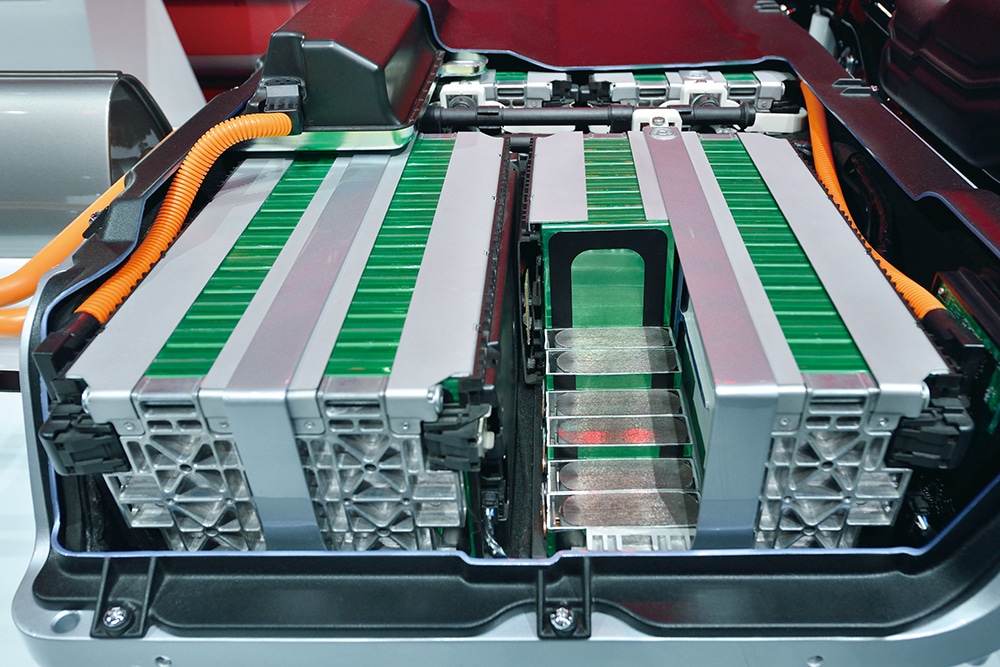
Why do electric automobiles use lithium-ion batteries? The battery that powers our cell phones, tablets, computers, and other consumer devices is the same one that powers this. Electric cars are currently among the viable uses of lithium-ion that have developed over time. But remember that consumer electronics use a different type of lithium-ion than electric vehicles. Their applications still differ from one another.
Lithium cobalt oxide (LiCoO2) cathodes are the basis for the lithium-ion batteries that drive consumer gadgets. Why are they not utilized in electric vehicles? They are prone to thermal runaway when mistakenly overcharged or operating at high temperatures. Or, to put it another way, they burn. Electric vehicles and other power-intensive applications should not use this lithium-ion battery. Lithium iron phosphate (LiFePO4), which has a slightly lower energy density than LiCoO2 but is safer and has a longer lifespan, is the type of lithium-ion used in electric vehicles. Twenty years ago, the non-combustible LiFePO4 mixing formula was created.
Benefits of lithium-ion batteries for electric cars
For usage in electric vehicles, lithium-ion batteries provide several benefits over lead acid and nickel metal batteries. Higher energy density, longer cycle life, better energy efficiency, and no memory effects are some benefits (decrease in energy capacity after the battery is discharged).
- Higher energy density – Drivers concerned about range would want a battery that uses more power while lasting longer between charges. The lightweight lithium-ion is the best battery for usage in automobiles since it can store more energy. Lithium-ion does not waste energy since it maximizes each kilowatt per square inch it absorbs and stores.
- More excellent cycle life In comparison to lead acid and nickel-based batteries, a lithium-ion battery can survive between 2000 and 5000 charging cycles when adequately maintained.
- Low self-discharge — Rechargeable batteries and cells lose some of their maximum energy capacity over time and with repeated charges. Lithium-ion batteries have a substantially lower rate of self-discharge than nickel-based batteries.
- No priming is required. Some rechargeable batteries need to be primed before their initial charge. Not lithium-ion. It only needs one standard charge.
- Lithium-ion batteries don’t require periodic discharge or other comparable maintenance, in contrast to nickel-based batteries, which do. Lithium-ion batteries are almost maintenance-free and have no memory effects, much like the electric vehicles that store them.
- A lithium-ion battery can be charged quickly and effectively based on your charger. And your battery would continue to function correctly even if you didn’t get a full charge. Without worrying about a battery pack that isn’t fully charged, you can charge your electric car as much as you need for a trip.
Lithium-ion batteries’ drawbacks
Like any other technological advancement, lithium-ion batteries have some drawbacks.
- High cost of production – High battery cost translates to the high cost of electric vehicles. Lead acid and nickel-based batteries cost 40% less to make than lithium-ion batteries. However, as lithium-ion is employed more frequently in several applications, the price is anticipated to decline.
- Lithium-ion batteries need a battery management system (BMS) to prevent overcharging and short circuits because of how sensitive it is to how it is charged and drained. Thanks to this protection circuitry, the battery will retain voltage and function within safe parameters.
- Aging – Lithium-ion batteries lose some of their power capacity over time if they are not stored properly. It is the reason you park your electric vehicle outside the garage in the summer and inside during the winter.
Conclusion
How far the car industry has advanced is unknown. Nobody could have predicted that things would be as they are now. Each machine has excellent specs and working mechanics, distinguishing it from others.
In a nutshell, electric cars are currently a popular topic. Automobile lithium-ion batteries. They are practical as well as environmentally beneficial. The slogan vehicle is an electric vehicle, which is used to refer to anything with an electric motor.